The Atlantic humpback dolphin (Sousa teuszii) is one of the least understood coastal dolphin species, despite its dependence on nearshore habitats that bring it into close contact with human activities throughout its distribution range. Due to the lack of targeted field research on the species, information on the distribution, population size, ecology, behaviour, life history and mortality of the Atlantic humpback dolphin is scarce. The sections below provide summaries of what is known about the species, but there are significant data gaps and much of this information is based on studies of only a small number of animals.
Description
Atlantic humpback dolphins have a robust body shape, characterized by a distinct raised hump of connective tissue located midway along the back. A small dorsal fin with a rounded tip is situated on top of the hump. The species has a well-defined long and slender beak; the lower jaw is paler grey in coloration than the upper jaw. The flippers are broad, with a straight trailing edge and rounded tips. The overall coloration is grey (but appearing brown-grey to black depending on light conditions), with the pigmentation grading from a darker grey dorsal cape to a light grey ventral surface and throat. In some animals the tailstock is a silvery grey covered with dark oval flecking. A darker grey eye patch is present. Calves have a lighter grey body coloration than adults. Atlantic humpback dolphins reach maximum body lengths of approximately 2.8 m. Adult males are larger, more robust (with a deeper tailstock keel), and have a more pronounced dorsal hump, than females. The hump and dorsal fin of some larger adults may be bordered by white pigmentation. network of local partners and national contact points in all of the confirmed and potential range states.

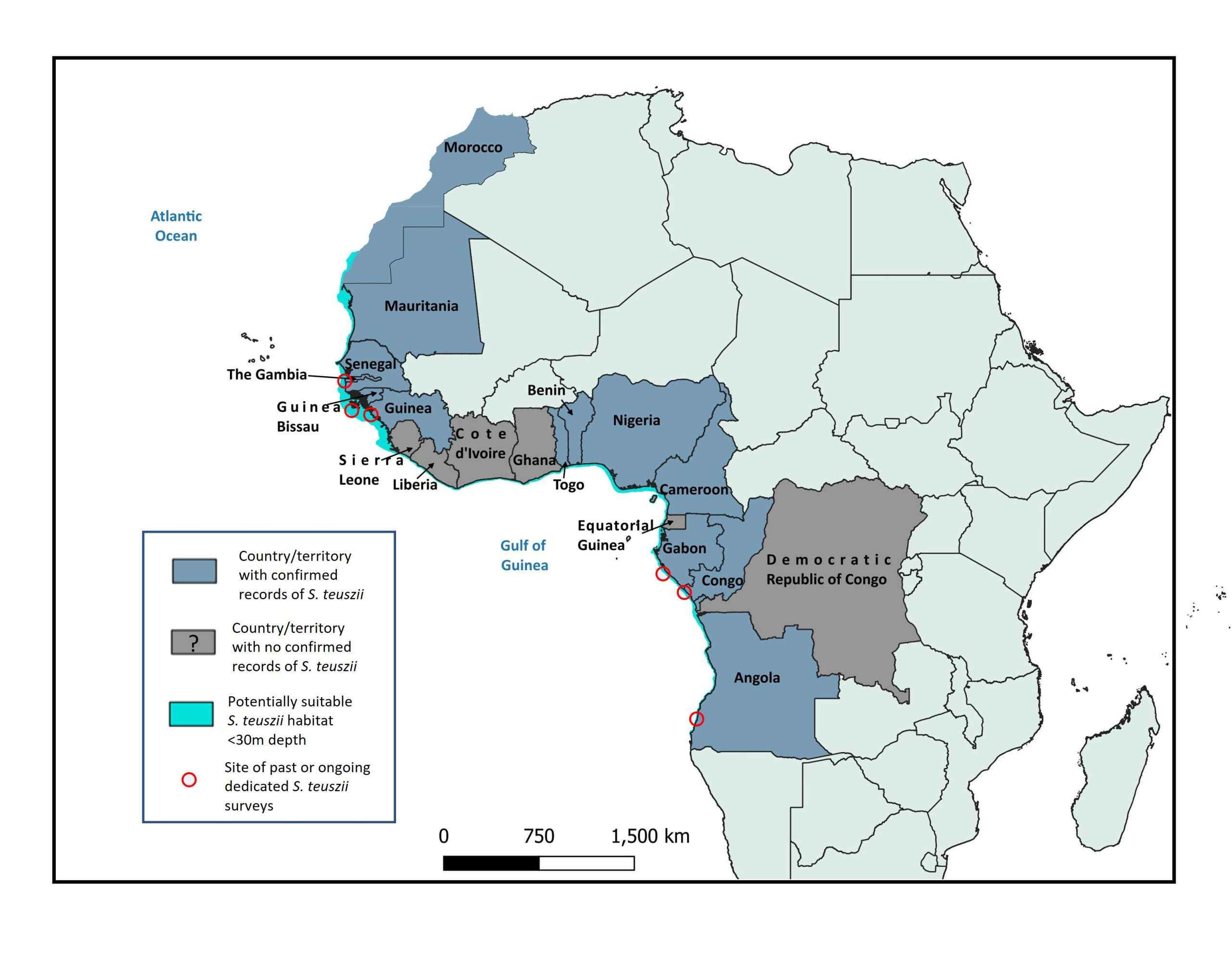
Distribution
Atlantic humpback dolphins are endemic to (sub)tropical waters along the Atlantic African coast[1-3]. In most countries, their occurrence is known only from opportunistic sightings, strandings, or bycatch reports. Together with a small number of targeted field studies, such records have documented Atlantic humpback dolphins in 13 range states to date, comprising (from north to south): Dakhla Bay south of Morocco; Mauritania, Senegal, The Gambia, Guinea-Bissau, Guinea, Togo, Benin, Nigeria, Cameroon, Gabon, Republic of Congo, and Angola[1-3]. The latitudinal limits are 23°54.5’N[4] and 15°38.4’S[5]. Their occurrence in an additional six countries within that broad latitudinal range (Sierra Leone, Liberia, Côte d’Ivoire, Ghana, Equatorial Guinea, and the Democratic Republic of the Congo) remains unconfirmed. It is unclear whether the absence or scarcity of records in many confirmed or potential range states is due to lack of observation effort and reporting, genuine rarity of the species, or reflects a discontinuous distribution range (either due to suboptimal habitat, or local extirpations from unsustainable anthropogenic pressures). The species does not occur around offshore islands separated from the mainland by deep water, such as Bioko.
Abundance
No robust population size estimates exist for Atlantic humpback dolphins in any range state[1, 2, 6]. It has been inferred from anecdotal data and ‘guesstimates’ that the species’ total abundance within its geographic range may be below 3000 animals[1], and that value was used for the IUCN Red List assessment in 2017[7]. Additionally, population structure has not been studied and requires clarification. Photo-identification work has yielded ‘minimum estimates’ of the number of dolphins in three small (relative to the total country coastline length) study areas: 103 individuals in the Saloum Delta region of Senegal[8], 47 animals in an approximately 25 km × 15 km study area in the Río Nuñez region of northern Guinea[9], and 10 animals along 35 km of coast in southern Angola[5]. Those studies had limited temporal and spatial extents, and, with the possible exception of the Angolan work, are unlikely to have photographed all of the animals using those areas. While encounter rates are available for a number of studies (including those above, and work in Gabon and the Republic of Congo[10]), they are not directly comparable due to different methods (e.g. platforms, study area extents and seasons).

Examples of individual Atlantic humpback dolphins with unique dorsal fin markings that can be used for photo-identification work.
Habitat
Atlantic humpback dolphins occupy a diverse array of nearshore habitats. In this context, ‘nearshore’ is defined as areas in which the sea floor is affected by wave motion, resulting in dynamic, tide-influenced, habitats. Consequently, potentially suitable habitat exists over larger areas and at greater distances from shore in geographic regions with shallow-sloping seabeds, such as the area between southern Senegal and Guinea[3]. Documented habitats include: large estuarine systems (including mangrove channels, upstream waters with tidal influence, and the estuary-influenced waters further offshore); exposed marine coasts (often within, or just beyond, the surf zone); coastal archipelagos; mud-flats, sandbanks and seagrass expanses; and large semi-enclosed bays[1-3, 11-14]. They predominantly occur shoreward of the 20 m depth isobath[3], and often in the shallowest (≤5 m depth) part of that range. Correspondingly, confirmed sightings have occurred within 13 km of the shoreline[3], including regular sightings in immediate proximity to the coast[2, 5, 8-11, 14-17]. Their northernmost and southernmost distribution appears to be broadly limited to mean annual water temperatures higher than 15ºC[3].

Examples of the different habitats occupied by Atlantic humpback dolphins, including close to shore along exposed marine coasts, several kilometers offshore, inside mangrove creeks, and in turbid estuarine-influenced waters.
Diet
As marine predators, Atlantic humpback dolphins potentially have substantial top-down ecological effects on nearshore ecosystems in areas where their populations are healthy. There have not been any targeted studies of their diet or interactions with prey species. Like other Sousa species, it is likely that Atlantic humpback dolphins are capable of feeding on a wide variety of benthic and pelagic fish, cephalopods and invertebrate species, and that important prey species vary between geographic areas and habitats.
Prey species confirmed during anecdotal sightings or from the stomachs of bycaught specimens include: grunts (Pomadasys spp.), including Sompat grunt Pomadasys jubelini[1, 18]; Bonga shad Ethmalosa dorsalis[19]; Gorean snapper Lutjanus goreensis[20]; Atlantic emperor Lethrinus atlanticus[20]; West African spadefish Chaetodipterus lippei[20]; Atlantic bonito Sarda sarda[5]; mullet Mugil spp. [19, 21], including South African mullet Liza richardsonii[5] and flathead grey mullet Mugil cephalus[8, 21]; Longneck croaker Pseudotolithus typus[1]; Cassava croaker Pseudotholithus senegalensis[1]; flounders Paralichthodes and Pseudorhombius spp.[1]; royal threadfin Pentanemus quinquarius[1]; and mantis shrimp Squilla mantis[1].

Atlantic humpback dolphins feeding on mullet in Angola (left) and Senegal (right).
Behaviour
The surfacing behaviour of Atlantic humpback dolphins usually comprises calm rolls, during which the beak is often lifted clear of the water and the body is arched, accentuating the hump. They appear to be a naturally unobtrusive species, preferring to maintain a distance from boat engines; however, they do occasionally leap, spy-hop and tail-slap. Travelling and foraging were the dominant behaviour reported during targeted focal follows of Atlantic humpback dolphins[5, 8, 9]. They may forage cooperatively to herd prey, sometimes trapping fish against the shoreline[5]. However, they also forage independently, during which individuals are more widely-dispersed, surface unpredictably, and sometimes exhibit tail-up dives[5, 8, 9].
Atlantic humpback dolphins typically travel in small groups; 65% of reviewed sightings comprised 10 or fewer animals, although larger groups of up to 45 individuals were reported[3]. Their distribution overlaps with bottlenose dolphins (Tursiops truncatus) throughout their range, and the two species have been observed travelling in mixed groups[5, 16]. Little is currently understood about the social affiliations or age/sex composition of Atlantic humpback dolphin groups, although there is evidence for strong social affiliation and stable group structure in some areas[5, 9].
Like other Sousa species, Atlantic humpback dolphins are capable of undertaking considerable spatial movements with the potential for relatively large home ranges. Swim speeds of 1–7 km/hr (mean of 4 km/hr) were recorded during travel along linear coastline in Angola[5]. Transboundary movements have been documented between some range states[2, 8, 10].

A small group of Atlantic humpback dolphins travelling along the coast (top), and examples of breaching, spy-hopping and surfing behaviour.
Vocalizations
Little is known of the acoustic behaviour of Atlantic humpback dolphins, but, like other dolphin species, they communicate and sense their surroundings through a combination of whistles, burst-pulse sounds and echolocation clicks. The only published study to date occurred in southern Angola[6, 22]. The dolphins produced broadband echolocation clicks, burst-pulse sounds, and whistles. The click trains had strongest energy above 10 kHz, with the highest energy levels occurring towards the 46 kHz upper frequency limit of the recording equipment, suggesting that the total frequency range (and probably also the peak frequency) of the clicks extended above that limit. Click trains ranged in duration from 0.53 to 37.80 s, with mean inter-click intervals of 43.3 clicks/s. The whistles were predominantly simple in shape, with over 85% having a single inflection point and most commonly being convex or concave in contour shape[6]. The mean minimum and maximum values of the fundamental frequency were 4.8 and 8.2 kHz respectively. At least one harmonic was present in 92% of whistles, and the harmonics of several whistles extended beyond the 46 kHz recording limit.
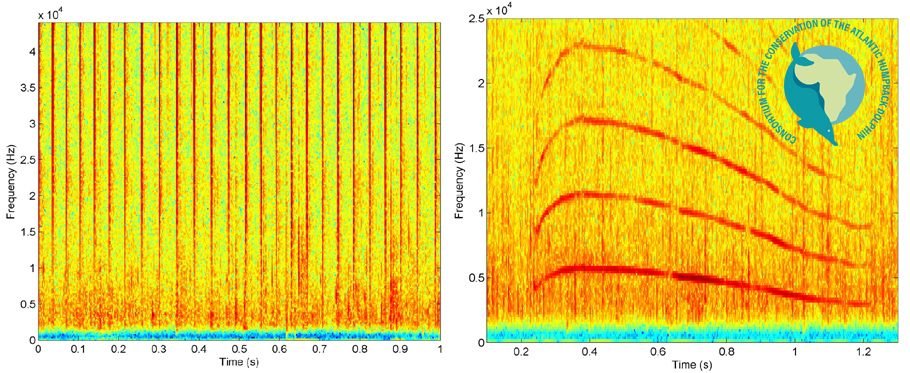
Examples of spectrograms (time versus frequency) showing the broadband echolocation clicks (left) and a whistle with harmonics (right) produced by Atlantic humpback dolphins in southern Angola. Adapted from Weir (2011).
Calving
Little is known about the reproductive or other life history parameters of Atlantic humpback dolphins, though they are likely to be broadly similar to those of other Sousa species, in which females reach sexual maturity at 9-10 years old, and produce calves at intervals of 3 to 5 years. In southern Angola, a calf was born during late April or May[5]. In the Saloum Delta in Senegal, a calving period was proposed during March and April[14]; however, at least three neonate calves (and many larger calves) were recorded during early November[8]. Consequently, calving may occur throughout the year with seasonal peaks, as in the Indo-Pacific humpback dolphin (Sousa chinensis[23]).

Young Atlantic humpback dolphin calves alongside their mothers in Angola (left) and Senegal (right).
Threats
Human threats and associated Atlantic humpback dolphin mortality rates have not been systematically studied, but include:
Immediate threats requiring action
- Bycatch (incidental capture) in fishing gear. Atlantic humpback dolphin mortalities from entanglement in (primarily) artisanal gillnets have been reported in multiple countries[1, 2, 13-16, 18, 19, 21, 24-31], and unsustainable mortality rates have been indicated in the Republic of Congo[25]. Dorsal fin injuries consistent with fishing line interactions have been documented in Guinea[9]. A mortality in a fish trap[20] and sightings of humpback dolphins in the vicinity of trawlers[9], indicates the potential for bycatch in other fisheries. Bycatch is widely considered to comprise the most prevalent immediate range-wide threat to the species[1, 2, 15, 28].
- Deliberate hunting. The use of meat obtained from strandings and bycatch[e.g. 2, 21, 25, 29] can evolve into directed hunts where animals are purposefully targeted as ‘marine bushmeat’ for human consumption[2, 32-34].
- Habitat loss and degradation. The deterioration or loss of habitat that supports dolphins or their prey can result from a range of human activities including coastal development (e.g. port construction), pollution (e.g. contaminants and runoff, oil spills, plastics), and dredging/trawling of seabeds.
Threats in need of assessment
These threats affect other dolphin species globally, but more research is needed to assess how they impact on Atlantic humpback dolphins:
- Prey depletion, due to overfishing by commercial and artisanal fisheries.
- Disturbance from tourism, including increasing boat-based dolphin-watching (e.g. Senegal and The Gambia) and kite surfing (e.g. Dakhla Bay).
- Disturbance from noise, including vessel noise, sonars, and seismic airguns
- Vessel strike, impact currently unknown.
- Climate change, potential impacts currently unknown.
- Live captures, not currently affecting the species.
Intensifying factors
Mitigation of potential threats is hindered by factors including the lack of available scientific data for, and awareness of, the species, increasing amounts of illegal fishing, and the high reliance of impoverished coastal communities on artisanal fishing for protein intake and livelihoods throughout the species range[1, 2, 7, 28]. Some of the potential threats may act synergistically, resulting in cumulative impacts on populations. Additionally, shifting baseline syndrome, where the accepted thresholds for environmental conditions are continually being lowered by progressive environmental degradation, is increasingly recognized as a fundamental obstacle to addressing conservation issues.

Conservation status
Atlantic humpback dolphins are considered particularly vulnerable to anthropogenic pressures, due to: (1) their restricted geographic range; (2) limited availability of their requisite ‘nearshore’ habitat; (3) low global population size; and (4) high spatial overlap with coastal human communities[1, 2, 7, 28]. The latter is particularly relevant given the widespread human poverty that occurs throughout the range of Atlantic humpback dolphins, with a high reliance on artisanal gillnet fishing for protein intake and livelihoods. The species is included on the Convention on International Trade in Endangered Species of Wild Flora and Fauna (CITES) Appendix I (Threatened), and since 2007 it has been included on the Convention on Migratory Species (CMS) Appendix I (endangered migratory species). In 2017, its conservation status on the IUCN Red List was uplisted from Vulnerable to Critically Endangered. Despite growing concern by scientists and wide acknowledgement of the likely declining conservation status of the species over the last two decades[1, 2, 28, 35, 36], little concerted progress has been made to date to implement conservation management measures for the species.

References
- Collins T. Re-assessment of the conservation status of the Atlantic humpback dolphin, Sousa teuszii (Kükenthal, 1892) using the IUCN Red List criteria. In: Jefferson TA, Curry BE, editors. Humpback Dolphins (Sousa spp): Current Status and Conservation, Part 1: Advances in Marine Biology. 72: Elsevier; 2015. p. 47-78.
- Van Waerebeek K, Barnett L, Camara A, Cham A, Diallo M, Djiba A, et al. Distribution, status, and biology of the Atlantic humpback dolphin, Sousa teuszii (Kukenthal, 1892). Aquatic Mammals. 2004;30(1):56-83.
- Weir CR, Collins T. A review of the geographical distribution and habitat of the Atlantic humpback dolphin (Sousa teuszii). In: Jefferson TA, Curry BE, editors. Humpback Dolphins (Sousa spp): Current Status and Conservation, Part 1: Advances in Marine Biology. 72: Elsevier; 2015. p. 79-117.
- Beaubrun PC. Un Cétacé nouveau pour les côtes sud-marocaines: Sousa teuszii (Kukenthal, 1892). Mammalia. 1990;54:162-4.
- Weir CR. Distribution, behaviour and photo-identification of Atlantic humpback dolphins Sousa teuszii off Flamingos, Angola. African Journal of Marine Science. 2009;31:319-31.
- Weir CR. Ecology and conservation of cetaceans in the waters between Angola and the Gulf of Guinea, with focus on the Atlantic humpback dolphin (Sousa teuszii): University of Aberdeen, U.K.; 2011.
- Collins T, Braulik GT, Perrin WF. Sousa teuszii (errata version published in 2018): The IUCN Red List of Threatened Species: e.T20425A123792572; 2017 [Downloaded on 08 March 2019].
- Weir CR. Atlantic humpback dolphins (Sousa teuszii) in the Saloum Delta (Senegal): distribution, relative abundance and photo-identification. African Journal of Marine Science. 2016;38:385-94.
- Weir CR. Photo-identification and habitat use of Atlantic humpback dolphins Sousa teuszii around the Río Nuñez estuary in Guinea (West Africa). African Journal of Marine Science. 2015;37:325-34.
- Collins T, Boumba R, Thonio J, Parnell R, Vanleeuwe H, Ngouessono S, et al. The Atlantic humpback dolphin (Sousa teuszii) in Gabon and Congo: cause for optimism or concern? Scientific Committee Report. International Whaling Commission, 2010 SC/62/SM9.
- Notarbartolo di Sciara G, Politi E, Bayed A. A winter cetacean survey off southern Morocco, with a special emphasis on right whales. Reports of the International Whaling Commission. 1998;48:547-50.
- Spaans B. Dolphins in the coastal area of Guiné Bissau. Lutra. 1990;33:126-33.
- Robineau D, Vély M. Les cétacés de Mauritanie (Afrique du nord-ouest). Particularités et variations spatio-temporelles de répartition: rôle des facteurs océanographiques. Revue d’Ecologie (la Terre et la Vie). 1998;53:123-52.
- Maigret J. Donnees nouvelles sur l’ecologie du Sousa teuszii (Cetacea, Delphinidae) de la cote ouest africaine. Bulletin de l’Institut Francais d’Afrique Noire. 1980;42:619-33.
- Ayissi I, Segniagbeto GH, Van Waerebeek K. Rediscovery of Cameroon dolphin, the Gulf of Guinea population of Sousa teuszii (Kükenthal, 1892). ISRN Biodiversity. 2014;2014:6. doi: doi.org/10.1155/2014/819827.
- Leeney RH, Weir CR, Campredon P, Regalla A, Foster J. Occurrence of Atlantic humpback (Sousa teuszii) and bottlenose (Tursiops truncatus) dolphins in the coastal waters of Guinea-Bissau, with an updated cetacean species checklist. Journal of The Marine Biological Association of the United Kingdom. 2016;96(4):933-41.
- Zwart SJ, Weir CR. Filling in the gaps: first record of Sousa teuszii in Benin (Gulf of Guinea: Africa). Marine Biodiversity Records. 2014;7:e59. doi: 10.1017/S1755267214000578.
- Cadenat J, Paraiso F. Nouvelle observation de Sotalia teuszii (Cétacé, Delphinidé) sur les côtes du Sénégal. Bulletin de l’Institut Français d’Afrique Noire. 1957;19:324-32.
- Cadenat J. Rapport sur les petits cétacés ouest-Africains. Résultats des recherches entreprises sur ces animaux jusqu’au mois de mars 195. Bulletin de l’Institut Français d’Afrique Noire. 1959;21:1367-409.
- Sequeira M, Reiner F. First record of an Atlantic humpback dolphin, Sousa teuszii Kukenthal, 1892 (Cetacea; Delphinidae) in Guinea-Bissau. Mammalia. 1992;56:311-3.
- Busnel RG. Symbiotic relationship between man and dolphins. Transactions of the New York Academy of Sciences. 1973;35:113-31.
- Weir CR. First description of Atlantic humpback dolphin (Sousa teuszii) whistles, recorded off Angola. Bioacoustics. 2010;19:211-24.
- Jefferson TA, Smith BD. Re-assessment of the conservation status of the Indo-Pacific humpback dolphin (Sousa chinensis) using the IUCN Red List Criteria. Advances in Marine Biology. 2015;73:1-26.
- Cadenat J. Notes sur les cétacés observés sur les cötes du Sénégal de 1941 à 1948. Bulletin de l’Institut Français d’Afrique Noire. 1949;11:1-15.
- Collins T, Stindberg S, Boumba R, Dilambaka E, Thonio J, Mouissou C, et al. Progress on Atlantic humpback dolphin conservation and research efforts in Congo and Gabon. Scientific Committee Report. International Whaling Commission, 2013 SC/65a/SM16rev.
- Segniagbeto GH, Van Waerebeek K, Bowessidjaou JE, Ketoh K, Kpatcha TK, Okoumassou K, et al. Annotated checklist and fisheries interactions of cetaceans in Togo, with evidence of Antarctic minke whale in the Gulf of Guinea. Integrative Zoology. 2014;9:1-13. Epub 2014/01/23. doi: 10.1111/1749-4877.12011. PubMed PMID: 24447657.
- Van Waerebeek K, Uwagbae M, Segniagbeto GH, Bamy IL, Ayissi I. New records of Atlantic humpback dolphin in Guinea, Nigeria, Cameroon and Togo underscore fisheries pressure and generalised marine bushmeat demand. Revue d’Ecologie (Terre et Vie). 2017;72:192-205.
- Weir CR, Van Waerebeek K, Jefferson TA, Collins T. West Africa’s Atlantic humpback dolphin (Sousa teuszii): endemic, enigmatic and soon Endangered? African Zoology. 2011;46:1-17.
- Cadenat J. Un delphinidae encore mal connu de la côte occidentale d’Afrique: Sotalia teuszii Kükenthal 1892. Bulletin de l’Institut Français d’Afrique Noire. 1956;18:555-66.
- Cadenat J. Observations de cétacés, siréniens, chéloniens et sauriens en 1955-1956. Bulletin de l’Institut Français d’Afrique Noire. 1957;19:1358-75.
- Bamy IL, Van Waerebeek K, Bah SS, Dia M, Kaba B, Keita N, et al. Species occurrence of cetaceans in Guinea, including humpback whales with southern hemisphere seasonality. Marine Biodiversity Records. 2010;3:e48.
- Alfaro-Shigueto J, Van Waerebeek K. Drowning in the sea of silence: the bushmeat concept applied for marine fauna. Fourth Biennial Zoos and Aquariums Committing to Conservation Conference; 28 November – 2 December 2001; Cocoa Beach, Florida2001.
- Leeney RH, Dia IM, Dia M. Food, pharmacy, friend? Bycatch, direct take and consumption of dolphins in West Africa. Human Ecololgy. 2015;43:105-18.
- Cadenat J. Observations de cetaces au Senegal. Notes Africaines. 1947;34:20-3.
- Jefferson TA. Endangered odontocetes and the social connection: selected examples of species at risk. In: Wursig B, editor. Ethology and Behavioral Ecology of Odontocetes: Springer Nature; 2019. p. 465-81.
- Reeves RR, Smith BD, Crespo EA, Notarbartolo di Sciara G. Dolphins, whales and porpoises: 2002-2010 conservation action plan for the world’s cetaceans. Gland, Switzerland and Cambridge, UK: IUCN/SSC Cetacean Specialist Group, IUCN, 2003.

